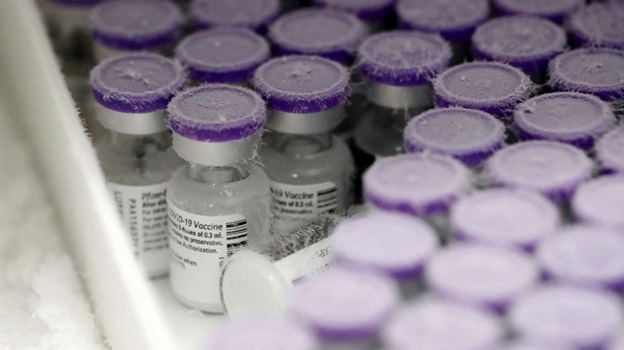
One of the most vital factors in maintaining vaccine efficacy is temperature-controlled storage, a process that ensures vaccines remain within their required temperature range from manufacture to administration.
This article explores how temperature-controlled storage preserves vaccine potency, why it’s essential, and the consequences of temperature deviations.
Understanding Vaccine Sensitivity to Temperature
Vaccines are biological products composed of weakened or inactivated pathogens, proteins, or genetic material designed to stimulate the immune system. These components are sensitive to temperature changes. If exposed to inappropriate conditions, either too hot or too cold, the vaccine can degrade or become inactive.
Common Temperature Ranges
- Refrigerated vaccines: 2°C to 8°C (36°F to 46°F)
- Frozen vaccines (like some COVID-19 types): –15°C to –50°C (5°F to –58°F)
- Ultra-cold storage (e.g., Pfizer-BioNTech COVID-19 vaccine): –60°C to –90°C (–76°F to –130°F)
Exceeding these thresholds can cause:
- Denaturation of proteins
- Loss of antigenic properties
- Reduced immune response upon administration
The Role of Temperature-Controlled Storage
Temperature-controlled vaccine cold storage refers to a continuous system that maintains optimal temperature conditions throughout the vaccine’s life cycle. This includes:
- Manufacturing facilities
- Warehousing
- Transport vehicles (air, land, sea)
- Local storage units (hospitals, clinics, pharmacies)
By ensuring consistency across all stages, the cold chain:
- Prevents degradation of active ingredients
- Preserves vaccine safety and efficacy
- Reduces waste and financial loss
- Ensures public trust in immunization programs
Technologies Used in Vaccine Cold Chains
To maintain temperature control, the cold chain relies on various technologies:
- Refrigerators and freezers: Special medical-grade units designed to maintain stable internal environments.
- Temperature data loggers: Devices that continuously monitor and record temperatures.
- Insulated shipping containers: Include phase-change materials or dry ice to keep vaccines cool during transport.
- Remote temperature monitoring systems: Allow real-time tracking and alerts for deviations.
- Backup power systems: Ensure continuous operation during power outages.
Consequences of Cold Chain Breakdowns
Even brief exposure to inappropriate temperatures can have serious consequences:
- Reduced potency: Ineffective vaccines may not produce immunity.
- Spoilage: Entire batches may need to be discarded.
- Financial loss: Replacing spoiled vaccines and repeated vaccinations increases healthcare costs.
- Public health risks: Poorly preserved vaccines can lead to disease outbreaks.
- Loss of public confidence: Reports of vaccine failure may undermine vaccination campaigns.
Best Practices for Maintaining Vaccine Potency
- Routine Temperature Monitoring: Check storage units at least twice daily.
- Staff Training: Ensure all personnel handling vaccines are trained in cold chain management.
- Regular Equipment Maintenance: Service refrigerators, freezers, and alarms regularly.
- Contingency Planning: Have emergency protocols for power outages or equipment failure.
- Documentation: Keep accurate records of temperature logs and vaccine handling.
Conclusion
Temperature-controlled storage is not just a logistical necessity, it’s a critical safeguard for public health. Preserving vaccine potency through rigorous cold chain management ensures that vaccines deliver their intended protection, preventing illness and saving lives.